主要信息
Target
Glutamine synthetase
Host Species
Mouse
Reactivity
Human, Mouse, Rat
Applications
WB, IF, ELISA, IHC
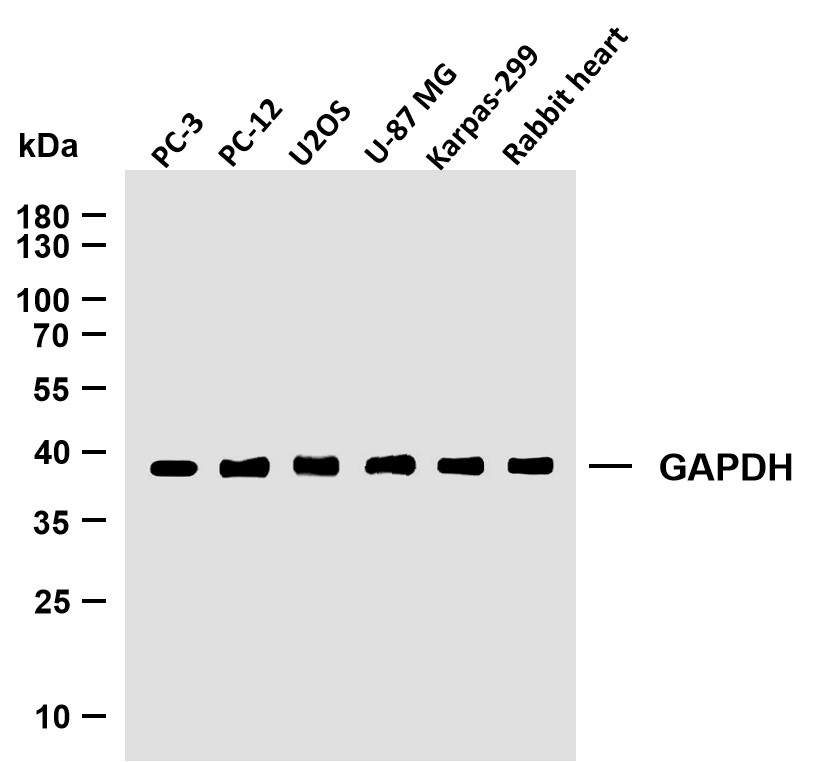
MW
42kD (Calculated)
42kD (Observed)
Conjugate/Modification
Unmodified
货号: YM4292
规格
价格
货期
数量
200μL
¥3,780.00
现货
0
100μL
¥2,300.00
现货
0
40μL
¥960.00
现货
0
加入购物车


已收藏


收藏
详细信息
推荐稀释比
IHC 1:200-1000; WB 1:500-2000; IF 1:100-500; ELISA 1:1000-5000;
组成
PBS, 50% glycerol, 0.05% Proclin 300, 0.05%BSA
特异性
This antibody detects endogenous levels of Gl Syn/Glutamine Synthetase protein.
纯化工艺
The antibody was affinity-purified from ascites by affinity-chromatography using specific immunogen.
储存
-15°C to -25°C/1 year(Do not lower than -25°C)
理论分子量
42kD
实测条带
42kD
修饰
Unmodified
克隆性
Monoclonal
克隆号
PTR1400
相关产品
抗原&靶点信息
免疫原:
AA range: 300-400
展开内容
特异性:
This antibody detects endogenous levels of Gl Syn/Glutamine Synthetase protein.
展开内容
基因名称:
GLUL
展开内容
蛋白名称:
Glutamine synthetase
展开内容
别名:
GLUL ;
GLNS ;
Glutamine synthetase ;
GS ;
Glutamate decarboxylase ;
Glutamate--ammonia ligase
GLNS ;
Glutamine synthetase ;
GS ;
Glutamate decarboxylase ;
Glutamate--ammonia ligase
展开内容
背景:
The protein encoded by this gene belongs to the glutamine synthetase family. It catalyzes the synthesis of glutamine from glutamate and ammonia in an ATP-dependent reaction. This protein plays a role in ammonia and glutamate detoxification, acid-base homeostasis, cell signaling, and cell proliferation. Glutamine is an abundant amino acid, and is important to the biosynthesis of several amino acids, pyrimidines, and purines. Mutations in this gene are associated with congenital glutamine deficiency, and overexpression of this gene was observed in some primary liver cancer samples. There are six pseudogenes of this gene found on chromosomes 2, 5, 9, 11, and 12. Alternative splicing results in multiple transcript variants. [provided by RefSeq, Dec 2014],
展开内容
功能:
Catalytic activity:ATP + L-glutamate + NH(3) = ADP + phosphate + L-glutamine.,Disease:Defects in GLUL are the cause of congenital systemic glutamine deficiency (CSGD) [MIM:610015]. CSGD is a rare developmental disorder with severe brain malformation resulting in multi-organ failure and neonatal death. Glutamine is largely absent from affected patients serum, urine and cerebrospinal fluid.,online information:Glutamine synthetase entry,similarity:Belongs to the glutamine synthetase family.,subunit:Homooctamer.,
展开内容
细胞定位:
Membrane, Cytoplasm
展开内容
组织表达:
Expressed in endothelial cells.
展开内容
信号通路
文献引用({{totalcount}})
货号: YM4292
规格
价格
货期
数量
200μL
¥3,780.00
现货
0
100μL
¥2,300.00
现货
0
40μL
¥960.00
现货
0
加入购物车


已收藏


收藏
Recently Viewed Products
Clear allToggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
主要信息
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
产品 {{index}}/{{pcount}}
上一个产品
下一个产品
{{pvTitle}}
滚轮缩放图片
{{pvDescr}}